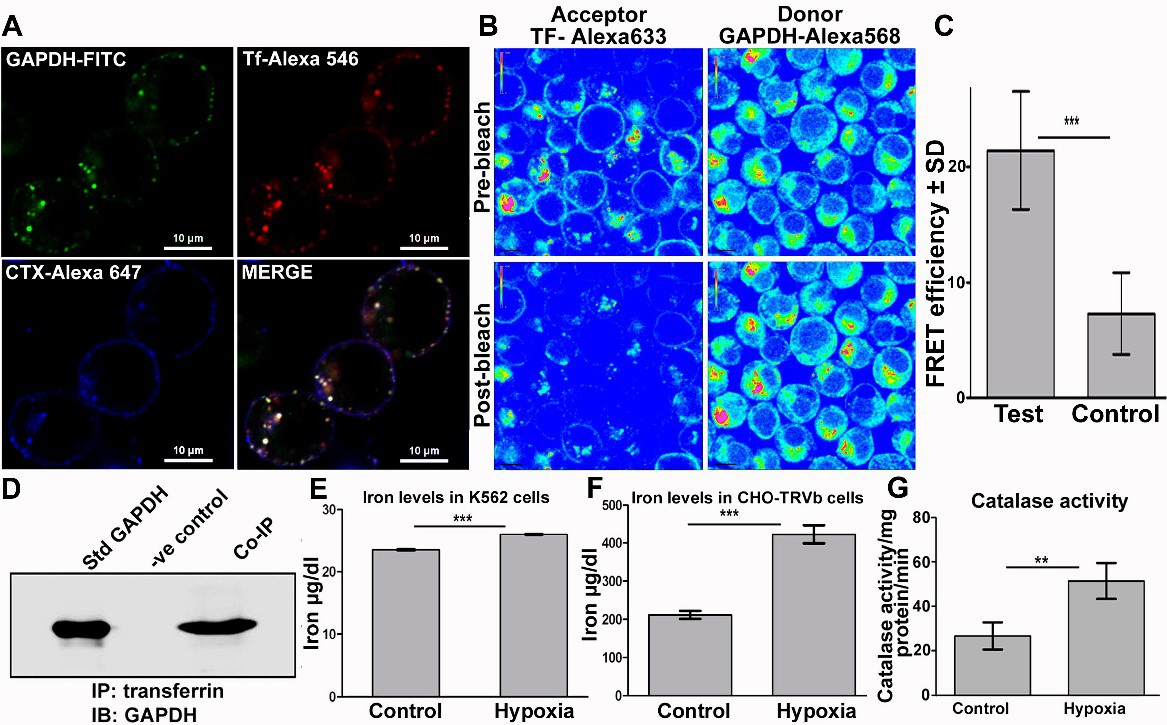
Fig. 3. Trafficking of transferrin by GAPDH under hypoxia delivers iron into cells. (A) Co-localization of Tf and GAPDH in plasma membrane rafts and endosomes of K562 cells after hypoxia exposure as observed by confocal microscopy, scale bar 10 Ám. (B) Tf - GAPDH interaction in the endosomes of K562 cells revealed by presence of a FRET signal which is represented by an increase in fluorescence of GAPDH-Alexa 568 (donor) upon photobleaching of Tf-Alexa 633 (acceptor). Image is presented in rainbow contrast mode to highlight the increase in GAPDH signal post bleaching of acceptor (lower right panel). (C) FRET efficiency was significantly higher than control sample, where instead of, labelled Tf an unrelated donor protein goat IgG-Alexa 633 was used. p<0.0001, n=58 test and n=20 control. (D) Co-immunoprecipitation confirms GAPDH-Tf interaction on plasma membrane of hypoxia exposed cells. (E&F) Cellular iron levels increase after hypoxia in both K562 & CHO-TRVb cells. For K562 cells p<0.05, n=3. For CHO-TRVb cells p<0.0001, n=3. The increase in case of K562 cells appears to be marginal but in fact is very significant as the newly recruited GAPDH receptor has to make up for the deficit caused by decrease in TfR1 expression (seen in Fig. 1D). (G) Exposure to hypoxia enhances cellular catalase activity, p<0.05, n=3.